
Immuno 101
IMMUNOTHERAPY
Game-Changer
Immunotherapy is a significant game-changer in the world of cancer treatments, and it is beginning to positively impact Cholangiocarcinoma patients. It has been said that 30 – 40% of all Cholangiocarcinoma patients have targetable mutations, that could match clinical trials.
“The discovery of the ‘Checkpoint Pathway and its Immunotherapy interventional drugs was a penicillin moment in the history of cancer treatment, we are now seeing the word cancer and cure in the same sentence for the first time.” Clinical Trials for Cholangiocarcinoma continue to increase.
Helping the immune system recognise and destroy cancer cells
Immunotherapy is a treatment that helps your immune system better recognise and destroy cancer cells. Immunotherapy can encourage your immune system to attack cancer cells. Immunotherapy can have side effects and you should discuss your options with your physician to understand how and if immunotherapy would be a treatment option for you.
The range of immunotherapy pathways is expanding
Most if not all immunotherapy options for Cholangiocarcinoma patients will fall under the Clinical Trial process. Please visit the Clinical Trial page to gain an overview of the trials available.
Let’s begin with the Checkpoint Pathway and their ICI – Immune Checkpoint Inhibitor treatments.
CHECKPOINT IMMUNOTHERAPY
The Checkpoint Pathway
T Cells have “Checkpoints” – failsafe switches on their surface, these checkpoints are called ligands – protein structures that protrude from the surface. Their job is to communicate with other cells and either prevent activation or deactivate an activated T Cell.
There are two primary “Checkpoint Pathways” one pathway activates and the other pathway deactivates.
- Activation of the T-Cell: This is when another cell called APC (Antigen-presenting cell) communicates to the T-Cell by presenting evidence of a foreign unknown threat (Cancer or a virus) it essentially presents a piece of the cancer (or virus) cell to the T-Cell – much like a piece of clothing for a bloodhound to gain the scent. This communication activates the T-Cell to seek and destroy the target. A Checkpoint ligand on the surface of a T Cell called “CTLA4” can prevent this activation from occurring.
- Deactivation of a T-Cell: This is when the activated T-Cell locates its target, but before it proceeds with the attack it communicates with the target cell (cancer cell) to ensure that it is in fact a cancer cell. A Ligand on the surface of the T-Cell called PD1 can abort (deactivate) this attack if it thinks the cell is in fact “healthy.” Cancers can trick this process by expressing a corresponding friendly ligand called PD-L1 on its surface. A PD1/PD-L1 communication binding deactivates the T-Cell attack.
Dr. James Allison 2018 Nobel Prize Winner demonstrated that blocking/inhibiting these checkpoints would ensure the T-Cells would be activated or remain activated and therefore continue on to destroy the targeted cancer cells.
So to summarise
- There are “Checkpoints” (CTLA4) on the surface of T-Cells that prevent them from being activated
- There are “Checkpoints” (PD1) on the surface of T-Cells that deactivate an activated T-Cell attack.
- When PD1 binds with PD-L1 the T-Cell is deactivated and the tumour can continue to grow.
Dr. James Allison 2018 Nobel Prize Winner demonstrated that blocking/inhibiting T-Cell checkpoints would ensure they would be activated and or remain activated to destroy the targeted cancer cells.
Overview of T-Cell Checkpoints
- There are “Checkpoints” (CTLA4) on the surface of T-Cells that prevent them from being activated
- There are “Checkpoints” (PD1) on the surface of T-Cells that deactivate an activated T-Cell attack.
- When PD1 binds with PD-L1 the T-Cell is deactivated and the tumour can continue to grow.
The battle to activate or prevent activation of a T-Cell
The image above shows CTLA4 being blocked by a monoclonal antibody (Y shaped) from interacting/binding with the B7 ligand. This binding would prevent the T-Cell from being activated. Activation only takes place if CD28 successfully binds with B7. If CTLA4 becomes dominant it outperforms CD28, by blocking CTLA4 CD28 will be successful and activate the T-Cell.
The battle to deactivate and activated T-Cell

The image above shows a tumour cell expressing a PD-L1 ligand and attempting to bind with the T Cells PD-1 checkpoint Protein. A successful binding will result in the PD-1 switching off the T Cell attack. The image also shows the presence of the “Checkpoint inhibitor drug” Keytruda’s monoclonal antibody (Y Shaped) intercepting and blocking this binding /connection.
What are Immune Response T Cells
T Cells are relentless and very efficient killing machines, but they need to see the threat. When the immune system activates its T Cell army against a new threat, the activated T Cells express a checkpoint protein called PD-1 onto their surface
What are PD-1’s
PD-1 is an Immune inhibitory checkpoint molecule that is expressed by an ”Activated” T Cell. The PD-1 function is to bind and communicate with PDL-1 receptors that sit on the surface of healthy cells. When PD-1 successfully binds with a PD-L1 ligand-protein, it then transmits an inhibitory signal back to the T Cell to cease the attack.
What is PD-L1
PD-L1 Ligand is an elongated cluster protein that a cell expresses. PD-L1 ligands attach themselves to the membrane surface of a healthy cell. It’s function is to act as an immune regulatory molecule (A Check Point Molecule) that protects healthy cells from being attacked by T Cells.
The immune System has Checkpoints
Our immune system is essentially in a permanent attack mode and has inbuilt checkpoints (PD-1) to switch off attacks when encountering healthy Cells. Tumours have learnt to exploit this by expressing the PD-L1 ligand on their cell surface.
Do you qualify for Checkpoint Immunotherapy?
It all begins with your biopsy (tissue sample) being tested for specific biomarkers that indicated that this type of treatment has a chance of working. A simple time & cost-efficient IHC test (Immunohistochemical) takes 3 – 5 days. This is not the same as a Molecular test. Learn more about IHC test
Biomarkers are essentially known and measurable characteristics that are found in your tissue sample. These known biomarkers can be subjected to an IHC staining test that informs your oncologist as to whether you have the right biomarkers for checkpoint immunotherapy. An Example biomarker PD-L1: if this is found in your tissue sample, it is known to bind with the checkpoint PD1 which causes deactivation of the T-Cell. This biomarker information informs your oncologist that this could be a viable reason for the growth of your tumour(s). This would be a strong indicator to include immunotherapy into your treatment plan. Please note that there must be a higher concentration of PD-L1 in your biopsy sample for this to proceed.
An IHC test will also reveal other required biomarkers such as MSi-high (Microsatellite Instability) which is also a clinical trial requirement. As part of our immune system, we have a “Spell Checker” process – a set of 4 genes called MMR – (mismatch repair genes) whose job is to ensure that any DNA replication mistakes are corrected immediately. When this process fails ie one or more of the MMR genes goes silent then mistakes go unchecked and cancers potentially begin to grow. When this MMR process fails or is faulty it is referred to as dMMR (Deficient MMR). It is known that this deficiency causes microsatellite instability, if this instability is high it will more likely be the cause of mutations and cancerous clusters.
So to summarise – an IHC test can discover if your tumour contains 2 biomarkers PD-L1 and MSi-High, it is believed that if you have both biomarkers, then you are more likely to benefit from a checkpoint immunotherapy treatment.
- If an IHC test does not yield a favourable match then ask your oncologist to order a full molecular (genomic) profiling of your biopsy.
- If you do not have a biopsy – tissue sample of your primary tumour, ask your oncologist to explore all opportunities to gain one.
Microsatellite Instability (MSI)
- Microsatellites are parcels of DNA code
- Microsatellite Instability in those parcels (MSI)
- Mismatch Repair (MMR) is our body’s DNA spell checker
- Deficient Mismatch repair (dMMR) means failure of the spell checker
MSI
Microsatellite Instability occurs when the strings of DNA contained within the microsatellites, replicate incorrectly creating errors in our DNA code.
MSI is described as follows
- MSI-high: (MSI-H) occurs if two or more microsatellite markers show instability
- MSI-low: (MSI-L) occurs if there is only one marker unstable
- MSI-Stable : (MSS) results when all the five microsatellite markers are stable
Reporting often describes MSI as follows
- MSI-h > 30%
- MSI-L <30%
- MSS – 0% instability
MMR the Spell Checker
- MMR – means all genes are functioning / expressing correctly
- dMMR – when one or more genes are not expressing and correctly
MMR
There are 4 primary MMR genes are MLH1, MSH2, MSH6, and PMS2
Think of these genes as our body’s spell checkers, their job is to express proteins that fix/repair DNA replication errors as they occur. This is a normal function within our body.
What is spell checking?
- Errors occur often in our DNA replication process.
- Microsatellites are parcels that contain strings of our pre-made dna code – the code that makes us – us!
- This replication process is constant and errors are normal
- When all 4 MMR genes are expressing proteins (spell checkers), then this is described as MMS (Stable).
dMMR
When one or more of these genes fail to express their proteins (spell checkers) this means the MMR process is now deficient and is not correcting all DNA mistakes as they occur. This then leads to the accumulation of DNA replication errors within the microsatellites creating microsatellite instability.
Uncorrected errors lead to accumulations of defective cells that cluster and can form cancer mutations.
Immunotherapy and MSi-High
The MSi-high status has so far proven to perform best with Checkpoint Inhibitor Immunotherapy treatments, so this is the primary testing objective.
Testing for MSi-high
There are 2 primary testing methods to determine this, both require a tissue sample (biopsy)
- IHC fast efficient and inexpensive
- Molecular profiling more expensive and slower
- Blood Profiling – Still in early stage
IHC and Molecular test accuracies a 92% and 95% with blood much lower at this stage (2019)
For the purpose of discovering if you are a match for Check Point Immunotherapy, the IHC test is most logical. If via the IHC test you do not have the MSi-high as a biomarker then pursue the full “Molecular Profiling” to discover a full road map of your tumors make up and what mutations are driving your cancer. This can then be matched up with known treatments globally.
IHC Test Description
Immunohistochemistry Test – fast and Efficient (3 -5 days) see Testing Page
What you testing for is MSi-high result, as immunotherapy responds well to this.
What do Microsatellites do?
They are the containers that hold our repetitive sequences of nucleotides (DNA code).
What are Nucleotides?
- Nucleotides are sequences of our DNA code. (Sequence repeats)
- Nucleotides are our “DNA Building Blocks”
- Nucleotides typically have 2 to 7 different types of pre programmed sequences of repeat code –
- Each sequence can contain between 2 -25 characters (string or stretch of code) – see diagram below.
- Nucleotide sequences continually replicate.
- Everyone has different pre programmed sequences.There can be hundreds of different code sequences.
The Nucleotide replication process can often go wrong
The MMR expressed proteins correct these spontaneous errors as they occur. When our MMR spell Checker is compromised or weakened then inconsistencies in the Microsatellites Nucleotides repeats over and over, this is when cancers can form.
Lab testing (IHC) can easily discover this when comparing healthy cell numbers or sequences, with cancer cells. If a difference is identified, then this is referred to as “microsatellite instability”.
MSI levels/identification
Image
Microsatellite Markers
Samples of Nucleotide code sequences contained within a microsatellite are supposed to replicate exactly the same in tandem.

Scott Paulson MD
Extract from CURE who spoke with Scott Paulson, M.D. medical oncologist with Texas Oncology, an affiliate of The US Oncology Network, to break down exactly what MSI-H status is, and the effects it may have on certain malignancies. Full Article link
MSI-high tumours form a well-defined group with distinct clinicopathologic features (more easily identifiable) which characterizes an overall better long-term prognosis.
MSI-H is a feature of cancer’s genetic coding, which results in it behaving and “looking” a certain way on a microscopic level. Due to defects in the way that DNA in the cancer cells repairs itself, it creates changes and mutations to normal body cells that can eventually let them turn into cancer. However, these cells become so abnormal, because of this feature, that the immune system, which is used to protecting the body against bacteria and viruses and other foreign invaders, can actually look at the cancer and recognize that something is very wrong. This can call the body’s normal defenses against invaders to try to attack the cancer.
TMB is also a factor
Something to be aware of.
When the body has a dMMR/MSI-High environment it typically sees a higher TMB (tumour mutation burden) in the body and these tumours also have a strong correlation to higher expression of the protein-ligand PD-L1 which anchors to the surface of the tumour cells.
Tumor Mutation Burden (TMB) refers to the total amount of cancer tissue in the body. The survival of a patient relates to the tumor mutation burden, disease location and most importantly, the pace of the disease. In general, patients with a high burden of tumors have a high pace of disease and therefore a very short survival without therapy.
More About TMB
Source: Onclive 2018
Patients most likely to respond to immune checkpoint inhibitors (ICIs), tumor mutational burden (TMB) has emerged as a highly promising and clinically validated biomarker. Results from studies have demonstrated that subsets of patients with high TMB exist across almost all cancer types and that assessing TMB through whole-exome sequencing (WES) or next-generation sequencing (NGS) can predict response to a range of different types of immunotherapy.
TMB has been a hot topic at oncology conferences this year. Investigators and test developers must overcome a variety of challenges before it is ready for prime time as a biomarker, but it appears poised to bring immunotherapy into the era of personalized medicine.
A Heavy Load
It is a central tenet of cancer biology that tumors arise and evolve as a result of the acquisition of damage to the genome, generating characteristic genomic alterations that lead to the dysregulation of key cellular processes termed cancer hallmarks. In addition to identifying individual frequently altered genes that function as drivers of particular cancer types, results from genome sequencing studies have revealed the global spectrum of somatic mutations across a given tumor, known as the TMB or mutational load. TMB is defined as the number of mutations per megabase (Mb) of DNA.
Seeds of Their Own Destruction
It has long been suspected that cancers with a greater number of gene mutations may provoke a stronger antitumor immune response. The thinking behind this hypothesis relates to the production of neoantigens—fragments of proteins expressed on the surface of cancer cells that are encoded by mutated genes. Neoantigens are unique to cancer cells because they are derived from a mutant gene, which may encode a mutant protein that differs from that expressed by normal cells. Therefore, neoantigens have the potential to be recognized as foreign by the cells of the immune system that patrol the body. A greater number of neoantigens might mean increased stimulation of those immune cells and stronger immune response. In this way, cancer cells may generate the seeds of their own destruction.
My Kingdom for a Biomarker
MSI and dMMR and are just 2 of the many phenotypes that can cause hypermutant tumors and contribute to high levels of TMB. If tumors with higher TMB provoke a stronger immune response, then it stands to reason that TMB could provide a means for more comprehensive assessment of patients who might respond to ICIs and potentially other immunotherapies. In the past several years, investigators have started to evaluate TMB in this capacity, and it has emerged as a powerful predictor of response to ICIs in a range of tumor types Investigators are seeking to correlate TMB with clinical and biological outcomes in a number of ongoing clinical trials.
High TMB
Source: Caris life Sciences
Tumor Mutational Burden (TMB) is an emerging, quantitative indicator for predicting response to novel immune checkpoint inhibitors across a wide spectrum of tumor types. TMB measures the total number of non-synonymous, somatic mutations identified per megabase of the genome coding area (a megabase is 1,000,000 DNA base pairs).
Tumors with high TMB likely harbor neoantigens and may respond more favorably to immune checkpoint inhibitors. Caris Molecular Intelligence defines TMB as 17 or more mutations per megabase. TMB is included for all MI Profile orders, at no additional cost, added tissue, or delay in turnaround time.
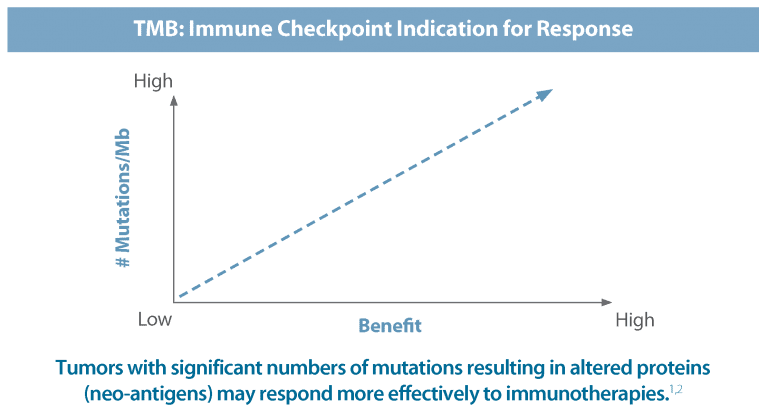
How Tumor Mutational Burden Works
- Non-synonymous mutations are changes in DNA that result in amino acid changes in the protein.
- The new protein changes result in new shapes (neo-antigens) that are considered to be foreign to the immune system.
- Immune checkpoint inhibitors are able to stimulate and allow the immune system to detect these neoantigens and destroy the tumor.
- Germline (inherited) mutations are not included in TMB because the immune system has a higher likelihood of recognizing these alterations as normal.

Healthcare investment analysts and media joined the Cancer Research Institute along with academic and industry leaders in the field for an intimate, invitation-only discussion called “Immuno-Oncology: A Future Look.” The event took place June 21, 2019, at the New York Academy of Sciences.
The panel of academic experts included 2018 Nobel Prize winner James P. Allison, Ph.D., of the University of Texas MD Anderson Cancer Center, Philip D. Greenberg, M.D., of the Fred Hutchinson Cancer Research Center, and Robert H. Vonderheide, Ph.D., D.Phil., of the University of Pennsylvania Abramson Cancer Center, all members of CRI’s Scientific Advisory Council.
An industry fireside chat featured insights and opinions from Bristol-Myers Squibb’s Vice President, Head of U.S. Oncology Awny Farajallah, M.D., FACP, and Regeneron’s Founder, President, and Chief Scientific Officer George D. Yancopoulos, M.D., Ph.D.
Meg Tirrell, biotechnology and pharmaceuticals reporter for CNBC moderated the industry panel and Jill O’Donnell-Tormey, Ph.D., CEO and director of scientific affairs at the Cancer Research Institute, moderated the academic panel and provided an overview of CRI’s mission, programs, and impact over the past 65+ years
Video via CCF Conference 2016
Dr.Kate Kelly – talks about immunotherapy (19mins mark)
She highlights keynote 158 (2015) the first-ever patient to gain a full and complete response (Rose)
OTHER IMMUNOTHERAPIES
A different approach, bypassing the Checkpoint Inhibitor.
Click here for Video explanations
What is the role of T-cells in the immune system?
T Cells are the soldiers of the immune system who search out and destroy foreign invaders, such as flu and cancer.
Chimeric antigen receptor CAR T Cell
- CAR T Cell Therapy is a way of engineering the body’s T Cells to recognise cancer, by reprogramming the body’s own T Cells to recognise and destroy the cancer.
- T Cells are removed from the patient and then the patient’s Tumour antigens are added to teach the T Cells.
- The newly modified T Cells are then grown into the millions and then reintroduced into the patient’s body.
- CAR T-cell therapy, is a new form of immunotherapy that uses specially altered T cells to directly and precisely target cancer cells.
The immune system is made up of a variety of cells and organs that normally protect the body from infection and cancer. An important component of the immune system is T cells, which have the capacity to hunt down and destroy abnormal cells, including some cancer cells.
Sometimes, cancer cells find ways to evade the immune system; so, the immune system needs to be retrained and enhanced to recognize and attack cancer cells. CAR T-cell therapy is one innovative approach to program and strengthens the immune system to attack some forms of cancer.
After a small portion of a patient’s own T cells has been collected from the blood, these cells are re-engineered in a special laboratory so they now carry special structures called chimeric antigen receptors (CARs) on their surface.
When these CAR T cells are reinjected into the patient, they multiply rapidly and these engineered receptors may help the T cells to identify and attack cancer cells throughout the body.
CAR T-cell therapy has been shown to be effective in B-cell acute lymphoblastic leukemia (ALL) and adult diffuse large B-cell lymphoma (DLBCL).
There are no approved CAR-T treatments worldwide for solid cancers. However, research is underway at Peter Mac in CAR-T cell treatment of solid cancers. Please see Clinical Trials.gov for further information.
Page Source https://www.petermac.org/car-t
More Coming soon
Coming soon
Common patient-reported side-effects
- Cold Sweats
- Temperature spikes
- Extreme fatigue
- Persistent Brain Fog
- Nausea and vomiting
- Poor appetite
- Weight loss
Video collections
Terminologies in Immuno 101
APC: Antigen Presenting Cells: The immunes messengers.
B Cells: learn the tumour or virus DNA to recognise if they return.
Biopsy: A tissue sample extracted from your tumor and used by a laboratory to discover Biomarkers.
CTLA-4: A Check Point protein that sits on the surface of the T Cells. They prevent mistake attacks on healthy cells.
Check Point Inhibitor– See PD-1 below
Car T Cells: An new innovating immunotherapy technique
IHC: Immunohistochemistry Test for discovering PD-L1 and MSi status
Ligand: An elongated strand of protein receptor that anchors to the cells surface. (ie PD-L1 is a Ligand receptor)
Molecular Profiling: Provides a DNA road map of the tumours fingerprint (Genomic) and your hereditary fingerprint (Genetic)
Microsatellites: Microsatellites are stretches of DNA that contain a repetitive sequence of nucleotides
Nucleotides: Nucleotides are the repetitive strings of DNA code that make us – us. example of a code string; “AAAAA or CGCGCGCG” (codes are short tandem sequences that replicate)
MSI: is a measure of Microsatellite Instability
MSI-LOW: is a low recorded measure of Microsatellite Instability.
MSI-HIGH: is a high recorded measure of Microsatellite Instability.
MSS: is Microsatellite Stable, which means no instability is present
MMR: Mismatch Repair is the DNA repair pathway that plays a key role in maintaining our genomic stability. MMR is our “Spell Checker” correcting any errors in our DNA replication process as they occur. MMR is made up of 4 proteins (MLH1, MSH2, MSH6, & PMS2 )
dMMR: Deficient Mismatch Repair means that one or more of the 4 MMR proteins absent and as a result, the MMR is not functioning correctly and is described as deficient
TMB: means Tumour Mutation Burden
PD-L1: means Programmed Death Ligand 1 – a cluster protein that generally coats and protects healthy cells from the immune systems T Cells
PD-1: is an Immune Check Point Inhibitor – its function is to switch off a T Cell attack. It does by binding and communicating with the PD-L1 anchored to the surface of a cell.
PD1/ PD-L Pathway: A communication pathway/channel that dampens or deactivates (switches off) an immune response – (T Cell attack) on healthy cells.
Names of Nucleotides. The five bases are adenine, guanine, cytosine, thymine, and uracil, which have the symbols A, G, C, T, and U, respectively. The name of the base is generally used as the name of the nucleotide, although this is technically incorrect.
Source: https://www.thoughtco.com/know-the-kinds-of-nucleotides-4072796
Also see this informative video
MLH1 and Lynch Syndrome
About MHL1
The MLH1 gene provides instructions for making a protein that plays an essential role in DNA repair. This protein helps fix errors that are made when DNA is copied (DNA replication) in preparation for cell division. The MLH1 protein joins with another protein called PMS2 (produced from the PMS2 gene), to form a protein complex. This complex coordinates the activities of other proteins that repair errors made during DNA replication. The repairs are made by removing a section of DNA that contains errors and replacing the section with a corrected DNA sequence. The MLH1 gene is a member of a set of genes known as the mismatch repair (MMR) genes.
Lynch Syndrome
About 50 percent of all cases of Lynch syndrome with an identified gene mutation are associated with inherited mutations in the MLH1 gene. Several hundred MLH1 gene mutations have been found in people with this condition. Lynch syndrome increases the risk of many types of cancer, particularly cancers of the colon (large intestine) and rectum, which are collectively referred to as colorectal cancer. People with Lynch syndrome also have an increased risk of cancers of the endometrium (lining of the uterus), ovaries, stomach, small intestine, liver, gallbladder duct, upper urinary tract, and brain.
MLH1 gene mutations involved in this condition prevent the production of the MLH1 protein or lead to an altered version of this protein that does not function properly. When the MLH1 protein is absent or nonfunctional, the number of DNA errors that are left unrepaired during cell division increases substantially. The errors accumulate as the cells continue to divide, which may cause the cells to function abnormally, increasing the risk of tumor formation in the colon or another part of the body.
Some mutations in the MLH1 gene cause a variant of Lynch syndrome called Turcot syndrome. In addition to colorectal cancer, people with Turcot syndrome tend to develop a particular type of brain tumor called a glioblastoma.
Another variant of Lynch syndrome, called Muir-Torre syndrome, can also be caused by mutations in the MLH1 gene. In addition to colorectal cancer, people with this condition have an increased risk of developing several uncommon skin tumors. These rare skin tumors include sebaceous adenomas and carcinomas, which occur in glands that produce an oily substance called sebum (sebaceous glands). Multiple rapidly growing tumors called keratoacanthomas may also occur, usually on sun-exposed areas of skin.
Source
https://ghr.nlm.nih.gov/gene/MLH1
Lynch Syndrome Website
https://lynchsyndrome.org.au/
My Experience
Keynote trial 158.
My basic Immunotherapy related details
dMMR: absent MLH-1 and PSM2 proteins
MSI-High
PDL-1 >49%
August 2017
I became a patient participant on a Global immunotherapy trial by Merck for a still experimental drug called Keytruda.
Immunotherapy has been quoted as being the modern day penicillin moment in history and a cure for cancer.
I had only one choice in my mind and that was to see this trial as an opportunity, the alternative was immanent and certain death. However immunotherapy does not work on everyone and is completely unknown with Cholangiocarcinoma which is more difficult and aggressive than many other cancers.
My experiences with Keytruda are available my Dairy “My Walk with Cholangiocarcinoma” under the Section “My Diary of Events” but in brief …
First infusion and 3 weeks
- 3 Weekly with scans every 9 weeks
- Infusions – just 30 minutes versus the 5 – 7 hour Chemo infusions
- Infusions are simple with no pain (unlike Chemo)
- Day 3: (Post first Infusion) all my tumour pain suddenly receded, and I was able to breath normally.
- Day 5: I fell ill and began seating profusely, heavily fatigued and unable to get out of bed. This was extremely worrisome and alarming with temperatures spiking frequently to 39.5 degrees. Claire was constantly on hand to apply ice and cold towels to bring my temperature down – this was most successful over a period of hours.
- Day 19 or 20: The immune response that had driven this dynamically broke and I arose a new person – I felt great, a little unbelievable that I could just snap back.
- Day 21: 2nd infusion – no reactions at all
Next 14 Months
- Fatigue becomes a constant, but much better than where I had come from
- Shortness of breath became a constant
- Itching hands, arms chest and scalp came in cycles
- I did have some markers that tested, such as thyroid and potassium, but remained well within tolerance levels for the trial
15 months of infusions
I mutually agreed to cease infusions as I was beginning to itch once again and it was agreed that Keytruda had well and truly done its job and Dr Matthew Burge drove home the point, that continuing on, would be unnecessarily exposing my body to potential harmful effects ie kidneys and other.
My trial details and response
- 9 Patients Qualified globally – I am one of the 9
- I achieved an immediate “Complete Full Response” that means all my tumours were eliminated.
- I am currently classified as “Persistent and Ongoing Full Response”.
- There have been 2 trials, Keynote Trial 028 in 2014 – (4 patients) with one complete response and Keynote Trial 158 – 2016 – (9 patients) with one complete response.
- Two patient participants over 2 trials in 4.5 years, have succeeded.
- Note there is also maverick patient in the mix – Matt Reidy from Virginia USA who did not qualify for a trial, but privately funded his own successful outcome. Matt is now 2 years post Keytruda infusions, we had the opportunity to meet in person in Salt lake City courtesy of CCF Matt and I keep in regular contact and have struck up a very good friendship.
I have learnt that there is an ‘Art’ to Living Life . . . . Read my story @ https://steveholmes.net.au
The Family that pulled me through




